Its the shape of these lattices that determine the minerals cleavage. How is cleavage related to a minerals atomic structure.
2 6 Mineral Properties Physical Geology 2nd Edition
The property of cleavage is useful in identifying a mineral species.

. Cleavage is the way in which certain minerals fracture along defined planes related to their atomic structure. Mica example-breaks in sheets every time. The directions of these surfaces are related to weaknesses in the atomic structure of the mineral and are always parallel to a possible crystal face.
It can display both. Form of crystal shape which is an external expression of a minerals internal atomic structure. Cleavage tendency of many minerals to split along definite smooth planar surfaces determined by their crystal structure.
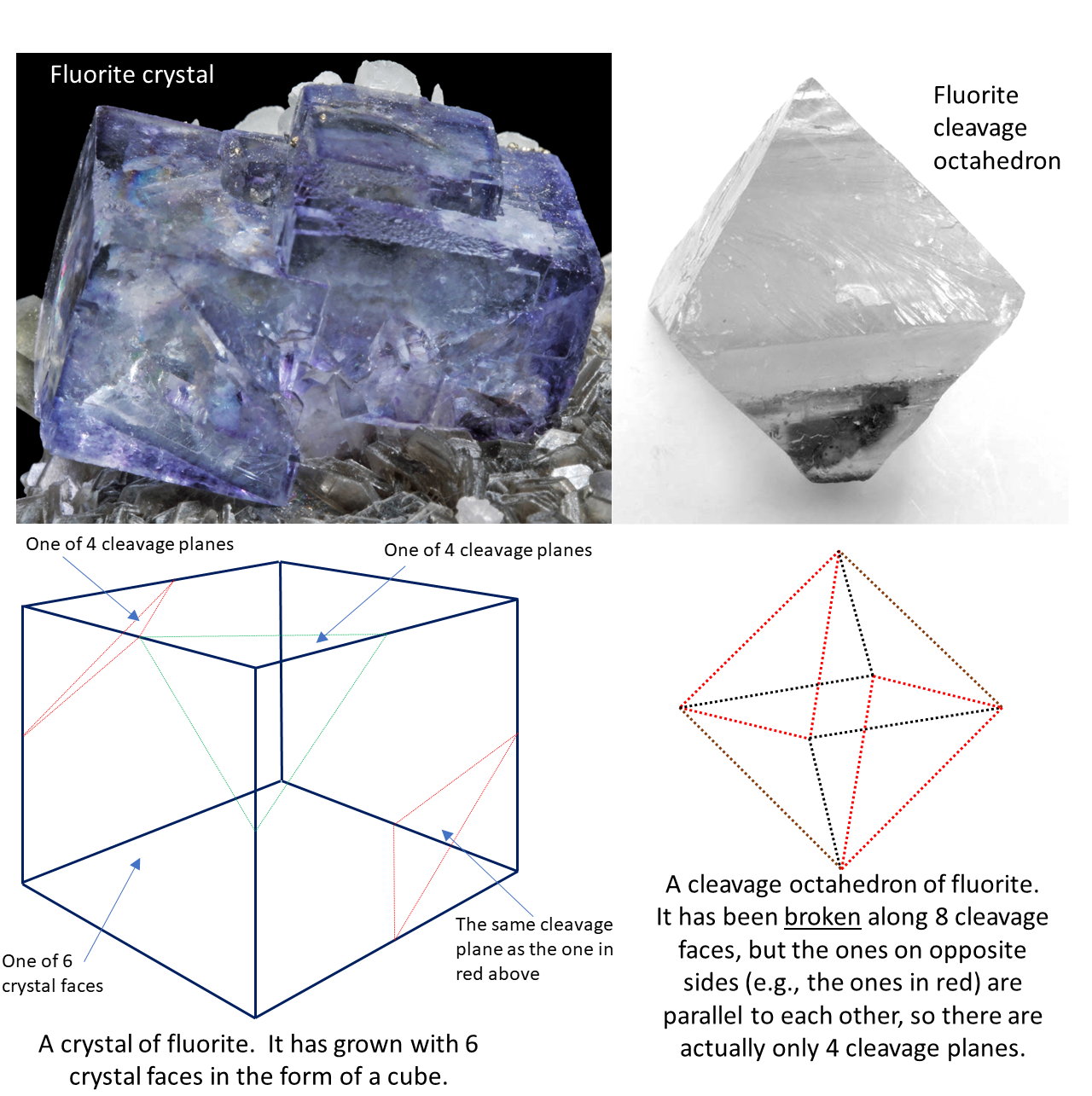
Six types of crystal shapes. Thin straight cuts on the cleavage plane. Cleavage exhibited on minerals of the isometric crystal system that are crystallized as octahedrons.
Cleavage-tendency for a mineral to break along the same planes of weakness-reflects internal arrangement of atoms. How is cleavage related to a minerals atomic structure. Cleavage exhibited on some prismatic minerals in which a crystal cleaves as thin vertical prismatic crystals off of the.
The directions of these surfaces are related to weaknesses in the atomic structure of the mineral and are always parallel to a possible crystal face. It helps for example distinguish some amphiboles from other similar minerals. Good distinct perfect 2.
Cleavage cleavage tendency of many minerals to split along definite smooth planar surfaces determined by their crystal structure. Cleavage can be an excellent diagnostic property. If the crystal grew with a dislocation in the lattice this may.
Explain three ways in which new minerals can form from existing minerals. The term cleavage refers to the way a mineral cleaves or breaks in prefered directions. A fracture can happen in any direction but usually a fracture will happen if it more energetically favourable.
Cleavage planes are usually to the crystallographic planes. Cleavage is related to the minerals atomic structure because minerals are arranged in crystal lattices. This means the cleavage is an easy plane to cleave-along meaning the mineral prefers to break along this plane.
What is the difference between atomic structure and crystal structure. Flat surface created from cleavage breakage. Cleavage is a growth plane in the mineral due to the structure of atoms.
Cleavage in silicate minerals reflects the arrangement of the silicon-oxygen tetrahedral. Of one another-same composition different mineral structure. Contrast the composition of minerals in each of the mineral groups discussed in the chapter.
Because cleavage is an external charactersitic to a minerals atomic structure. How is cleavage related to a minerals atomic structure. The physical properties of minerals comprise various measurable and discernible attributes including color streak magnetic properties hardness crystal growth form and crystal cleavage.
Surface created from breakage not related to atomic structure. -in the atomic structure of a mineral a set number of anions surround each cation--that. In this method of cleavage flat triangular wedges peel off of an existing octahedron.
The property of cleavage is useful in identifying a mineral species. Rule 1--coordination number principle. Each of these properties are mineral-specific and they are fundamentally related to a particular minerals chemical make-up and atomic structure.
Cleavage directions represent planes of weak bonding in the minerals atomic structure. Atomic structures of minerals Stable atomic structures in minerals can be explained largely by Paulings Rules 1-4 These rules assume atoms act as spheres and there is. An amorphous body of course has no cleavage.
Tendency of a crystalline mineral to break in certain directions yielding more or less smooth planar surfacesThese planes of lowest bond energy have minimum value of cohesion. Find step-by-step Earth science solutions and your answer to the following textbook question. Number is dependent on the ionic radius of the cation and anion--if the atoms are mostly.
How is cleavage related to the atomic structure. Spherical in shape ionic bond when combining spherical geometry can be used to derive the number. An example is Fluorite.
Tendency to break or separate along a flat surface due to a lack of or weakness in atomic structure.

Earth Archives On Instagram Anorthoclase Gets Its Name Is From The Greek An Or8os And Klasis Not Cleavi Minerals Minerals And Gemstones Positivity Crystals


0 Comments